U4AOS1 Topic 1: Introduction to Organic Chemistry
Introduction To Organic Chemistry
Organic Chemistry is the study of carbon-containing compounds, i.e, the stuff of life.
Technically we exclude some carbon-containing compounds such as oxides or carbonates from the study of Organic Chemistry, but that is not something we have to worry about.
Carbon has a valency number of 4, meaning that it must gain 4 electrons to obtain a stable electron configuration, hence it can form 4 covalent bonds with other C atoms or non-metal atoms. This ability to form 4 covalent bonds with other atoms is what gives carbon its unique versatility.
Carbon can have a single, double, or a triple bond with another Carbon atom.
If an organic molecule has ONLY single covalent bonds between its carbon atoms, we call that molecule ‘saturated’.
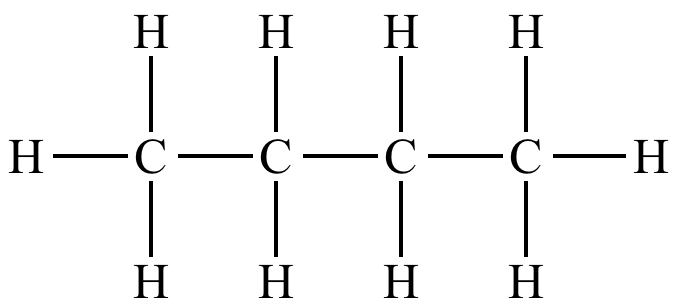
an example of a saturated molecule (butane)
If the organic molecule has one or more double/triple bonds between C atoms, we call it ‘unsaturated’.
 an example of an unsaturated molecule (ethene)
an example of an unsaturated molecule (ethene)
Carbon to Carbon covalent bonds have a relatively high bond strength, meaning that it takes a high amount of energy to break the covalent bond. Therefore, carbon compounds tend to be quite stable, partly explaining their prevalence on Earth. This stability generally means that these molecules are less likely to react.
(key words: less likely, these molecules of course can still participate in reactions, there's an entire topic called ‘organic reactions’!)
This table shows the relative strength of C-C bonds.
The covalent bonds that carbon forms with other elements can also be quite strong too, as shown in the table below.
Now considering big-picture organic molecules now rather than the bonds within them, it is important to know that these compounds can be expressed in 5 different ways as shown below.
VCAA expects you to know how to confidently draw/write out an organic molecule in each of the 5 ways.
These are the 5 ways in which the organic molecule Propanoic Acid can be expressed.
Another important introductory concept to note in Organic Chemistry is the prescence of isomers. Isomers are molecules that have the same molecular formula, but the atoms are arranged differently.
 <- these molecules both have the same molecular formula C4H10, but their atoms are arranged differently.
<- these molecules both have the same molecular formula C4H10, but their atoms are arranged differently.
The type of isomerism shown above is structural isomerism, where the atoms are connected in different ways. Another example is shown below.
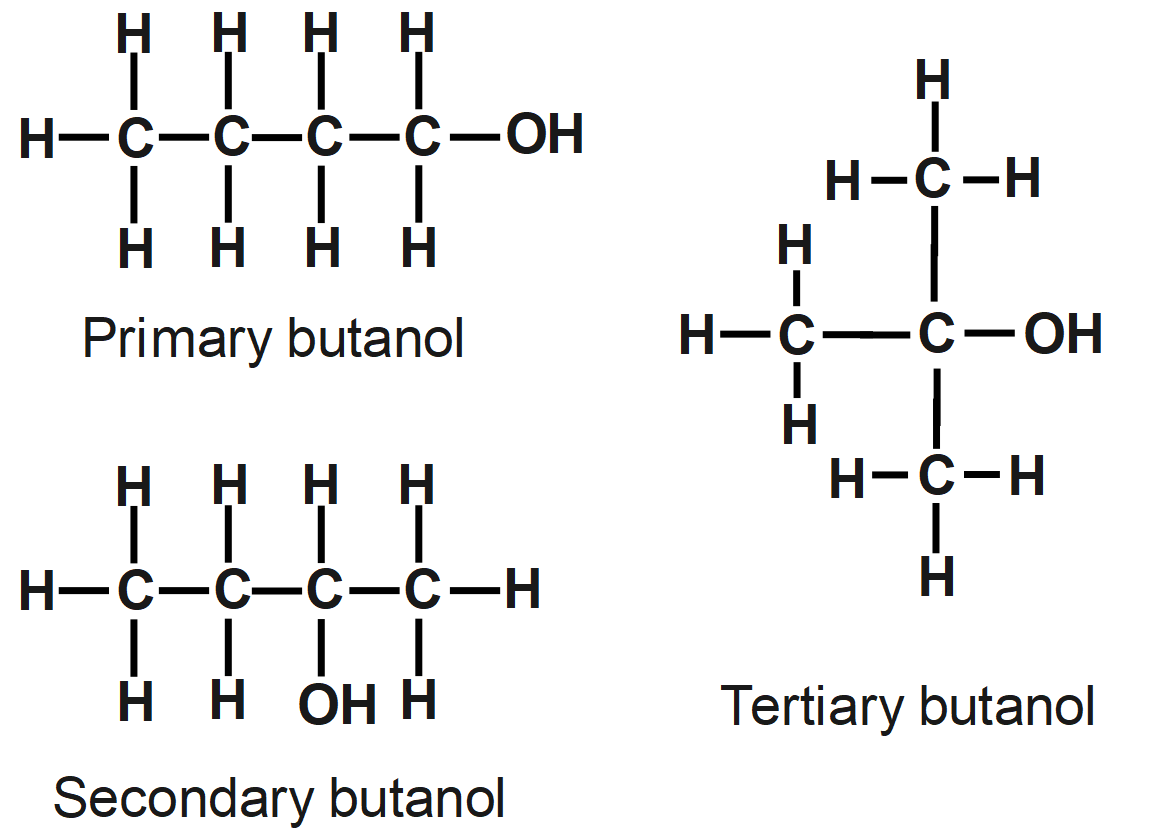
See how the position of the -OH group can change, or the branching of the Carbon chain? As the atoms are thus connected in different ways, these are all structural isomers of the same molecular formula C4H10O.
There is another form of isomerism that will be explored later called stereoisomerism where the atoms are indeed connected in the same way, however they have different arrangements in 3D space.
__________________________________________________________________________________________________________________________________________________________________________
Below is an interactive demonstration where you can try your hand at creating some organic molecules. The molecules you can create here are the ones you'll encounter frequently during your study of VCE Organic Chemistry.
Source: (JavaLab - Alkane Compound, https://javalab.org/en/alkane_compound_en/)